| Issue |
Dairy Sci. Technol.
Volume 89, Number 3-4, May-August 2009
1st IDF/INRA International Symposium on Minerals and Dairy Products
|
|
|---|---|---|
| Page(s) | 257 - 267 | |
| DOI | https://doi.org/10.1051/dst/2009006 | |
| Published online | 24 March 2009 | |
Original article
Thermodynamic characterization of calcium-milk protein interaction by isothermal titration calorimetry等温滴定量热法研究钙-乳蛋白质相互作用的热力学性质
Caractérisation thermodynamique des interactions entre protéines laitières et calcium par calorimétrie de titration isotherme
Latha-Selvi Canabady-Rochelle1*, Christian Sanchez2, Michel Mellema3 and Sylvie Banon1
1 Nancy-Université, INPL, École Nationale Supérieure d’Agronomie et des Industries Alimentaires, Laboratoire des Sciences et Génie Alimentaires, 2 avenue de la forêt de Haye, 54505 Vandœuvre-Lès-Nancy, France
2 Nancy-Université, INPL, École Nationale Supérieure d’Agronomie et des Industries Alimentaires, Laboratoire de Biocatalyse et de Bioprocédés, 2 avenue de la forêt de Haye, 54505 Vandœuvre-Lès-Nancy, France
3 Food Structural Design, Unilever Research and Development Center of Vlaardingen, Olivier van Noortlaan 120, 3133 AT Vlaardingen, The Netherlands
* Corresponding author (通讯作者): l.canabady@voila.fr
Received: 29 September 2008
Accepted: 13 January 2009
In milk, casein micelles are the natural vectors of calcium (Ca) and constitute an ideal vehicle for the delivery of additional Ca, through Ca-protein interactions. Isothermal titration calorimetry (ITC) is a technique of choice in the study of biomolecular interactions and especially in mineral-protein interactions. Calcium-milk protein interactions were thermodynamically characterized by titration of cow’s milk protein with calcium chloride (CC) using ITC. Experiments similar to that of ITC were reproduced and analyzed with other techniques (granulometry and electrophoretic mobility measurement) to better understand the nature of interactions. Binding titration curves were fitted with the “one set of sites model” to determine the thermodynamic parameters (N, 6 mol Ca·mol−1 of cow’s milk protein; K, 1070 mol·L−1; ΔH, 1910 cal·mol−1 of CC; and ΔS, cal·mol−1·K−1). Milk proteins bound up to 8 mg Ca·g−1 of protein at saturation. The global thermodynamic signal obtained upon titration was endothermic. The electrophoretic mobility variations of casein micelles occurring upon CC titration were indicative of Ca-protein interactions of electrostatic nature. Hence, the usual exothermic signal involved in electrostatic interactions was completely hidden by the strong endothermic signal coming from the release of water molecules, either from the hydration shell of the Ca ions and/or dehydration of hydrophobic core of proteins. Particle size variations were indicative of casein micelle retraction upon CC titration.
摘要
在乳中,钙与酪蛋白相互作用,使得酪蛋白胶束成为钙的天然载体和理想的传递者。等温滴定量热法是用来研究生物分子之间相互作用,特别是矿物元素-蛋白质间相互作用的一种有效方法。采用等温滴定量热法用氯化钙滴定乳蛋白质就可以研究钙-乳蛋白相互作用的热力学性质。本文同时还采用粒度测定和电泳迁移测定法进行了对比分析。根据测定的热力学参数(N, 6 mol·Ca·mol−1 乳蛋白; 束缚参数K, 1070 mol·L−1; 束缚热焓 H, 1910 cal·mol−1氯化钙; 熵S, cal·mol−1·K−1)确定的束缚滴定曲线符合“one set of sited” 模型。在饱和状态下每克乳蛋白可以束缚8 mg钙。根据滴定所获得的热力学信号显示钙的束缚反应为吸热反应。从氯化钙滴定酪蛋白胶束后电泳迁移的变化证明了钙-蛋白质相互作用的本质是静电作用。通常是由于静电作用所产生的放热信号完全被来自于钙离子水化层或者蛋白疏水核脱水作用释放出的水分子所产生的强吸热信号所掩盖。从粒度大小的变化结果表明随着氯化钙滴定度的增加酪蛋白胶束颗粒粒度减小。
Résumé
Dans le lait de vache, les micelles de caséines sont le vecteur naturel du calcium (Ca) et constituent le véhicule idéal pour délivrer le Ca supplémentaire, grâce aux interactions Ca-protéines. La calorimétrie de titration isotherme (CTI) est une technique de choix dans l’étude des interactions biomoléculaires. Les interactions Ca-protéines ont été caractérisées par CTI en titrant les protéines de lait de vache par du chlorure de calcium (CC). Des expériences similaires à celles menées en CTI ont été reproduites et analysées avec d’autres techniques (granulométrie et mesure de mobilité électrophorétique) afin de mieux comprendre la nature des interactions. Les courbes de titration ont été modélisées à l’aide d’un modèle à un seul site de fixation afin de déterminer les paramètres thermodynamiques (N, 6 mol Ca·mol−1 de protéines de lait de vache; K, 1070 mol·L−1; ΔH, 1910 cal·mol−1 de CC; ΔS, cal·mol−1·K−1). Les protéines de lait fixent jusqu’à 8 mg de Ca par g de protéines à saturation et le signal thermodynamique global obtenu lors de la titration est de nature endothermique. Les variations de mobilité électrophorétique des micelles de caséines se produisant lors de la titration en CC sont indicatives des interactions Ca-protéines de nature électrostatique. De ce fait, le signal exothermique induit par les interactions électrostatiques est complètement caché par un fort signal endothermique provenant du relargage de molécules d’eau, soit à partir de la coque d’hydratation des ions Ca et/ou de la déshydratation du cœur hydrophobe des protéines. La variation de taille des particules est indicative de la rétraction des micelles de caséines lors de la titration par le CC.
Key words: calcium / milk protein / interaction / isothermal titration calorimetry / nano-sizer
关键字 : 钙 / 乳蛋白 / 相互作用 / 等温滴定量热法 / 纳米粒度仪
Mots clés : calcium / protéine laitière / interaction / calorimétrie de titration isotherme / nano-sizer
© INRA, EDP Sciences, 2009
1. INTRODUCTION
Calcium (Ca) is a mineral that is essential for human beings especially during certain phases of life, such as growth (for bone formation), during lactation, and during old-age to act against osteoporosis. Yet most of the world-wide population do not satisfy their Ca needs. Even in the Western countries, specific populations at risk (girls from 10 to 19 years of age, women older than 56 years, and men older than 65 years) do not satisfy the recommended daily intake for Ca [31]. Calcium nutritional deficiency presents a world health challenge and can be overcome by Ca supplementation of food. More than 70% of dietary Ca comes from dairy products. Being naturally rich in Ca and with a high Ca bioavailability, dairy products are appropriate foods for Ca supplementation.
To the authors’ knowledge, few data are available concerning the thermodynamic characterization of Ca-milk protein interactions in an industrial mixture of proteins although the nature of the Ca-binding sites in cow’s milk protein have been often reported in the literature [10]. Isothermal titration calorimetry (ITC) is a technique of choice in the study of biomolecular interactions and especially in mineral-protein interactions [17]. Several studies reported the use of ITC for the thermodynamic characterization of Ca binding onto proteins, such as α-lactalbumin, calmoduline and lysozyme [11, 12, 16, 19, 24–26, 32]. Yet, ITC has never been applied to the study of Ca interactions into complex systems such as milk protein suspensions.
By means of ITC, thermodynamic characterization of the interactions is obtained in addition to the molar ratio of mineral binding onto proteins and to the binding constants [30]. ITC measures the binding equilibrium directly by determining the heat involved in the association of a ligand with its binding partner. In a single experiment, the value of the binding constant (K), the stoichiometry (N), and the binding enthalpy (ΔH) are determined. The free Gibbs energy (ΔG) and the entropy (ΔS) are determined from the binding constant. The enthalpy of the reaction upon Ca binding onto protein is either endothermic (ΔH > 0) or exothermic (ΔH < 0) and can be related to the nature of the reaction. An exothermic reaction is reported to characterize attractive interaction, mainly of electrostatic nature [28], and would be expected in the study of Ca-milk protein interactions. Unexpectedly, endothermic reactions are often observed upon Ca binding. Hence not Coulomb interactions, but rather liberation of water molecules from the hydration shells of the components is expected to be the driven energy source for the binding of multivalent ions onto polyelectrolytes [28].
The binding titration curve and the appropriate fit of experimental data determine whether there is one or more Ca-binding sites, independent or not, involving positive or negative cooperativity phenomenon. The aim of this study was to thermodynamically characterize the Ca-milk protein interactions. Indeed, in combination with structural information, the energetics of binding can provide a complete profile of the interaction.
2. MATERIALS AND METHODS
2.1. Milk reconstitution for ITC
For Ca-protein interaction study, cow’s skim milk was reconstituted in Milli-Q water from low heat skim milk powder (Ingredia, Arras, France) at 1% (w/w) protein concentration. As described in the industrial specification, low heat skim milk powder contains 35 g proteins, 1 g fat, 50 g lactose, and 8 g minerals for 100 g of powder. The milk was magnetically stirred for 3 h to enable mineral re-equilibration [1]. Then, the desalting step was performed on PD10 desalting column (Amersham Biosciences, Buckinghamshire, UK) to remove salts, which could interfere during Ca titration. According to their specification, former column removed up to 95% of salts, and the samples were stored at −20 °C until ITC analysis.
2.2. Protein concentration determination
Protein concentration was determined using Bradford quantification. Calibration curve was obtained using bovine serum albumin solution at 1.35 mg·mL−1, and various concentrations ranging from 0 to 1.35 mg·mL−1 were prepared. Then the proteins were dyed with Bradford reagent, previously diluted five-times (G250 100 mg, Bio-Rad S.A., Marne La Coquette, France; ethanol 50 mL; phosphoric acid 85% 100 mL, Labosi, Élancourt, France; distilled water 100 mL). At 20 μL of each point of the calibration curve and of the studied samples, 1 mL of diluted Bradford reagent was added, and the incubation time lasted for around 2–3 min. After agitation, absorbance was read at a wavelength of 595 nm on spectrophotometer (UV-160 A, Shimadzu Europa GmbH, Germany). Protein concentration, contained in the milk protein sample collected after the desalting step, was determined from calibration curve.
2.3. Calcium-milk protein interactions studied by ITC
VP-ITC microcalorimeter (MicroCal, North-Hampton, MA, USA) is composed of two identical spherical cells, a reference cell and a sample cell, both with a volume of 1.449 mL, which are enclosed in an adiabatic jacket. Before each experiment, both solutions were degassed for 7 min to eliminate air bubbles, which could falsify the baseline. Then, the sample and the reference cells were respectively filled with milk protein solution and with the solvent used to prepare the sample solution (i.e. Milli-Q water). The titrant, a 50 mmol·L−1-calcium chloride solution (CC, CaCl2·2H2O, Carl Roth GmbH, Karlsruhe, Germany) prepared in Milli-Q water, was injected stepwise into the sample cell. The measurement was performed under constant stirring (300 rpm) at a constant temperature (Texp = 25 °C). Small aliquots of the titrant (typically 2 or 5 μL) were successively injected into the solution of the working cell; 65 injections were performed (10*2 μL and then 55*5 μL). The total number of injections was set to reach milk protein saturation, and the spacing time was of 300 s. Because of possible dilution during the equilibration time preceding the measurement, the first injection was ignored in the analysis of the data. Each injection produced a characteristic peak in the heat flow due to the released or absorbed heat. Due to the lack of information about the expected heat of the studied systems, the reference power of the ITC instrument was set at 15 μcal·s−1, and each experiment was duplicated.
In the analysis, the titration of Ca solution into Milli-Q water was studied as the reference (i.e. ligand solution added to the solvent, without the presence of macromolecules), and this determined the heat of dilution of ligand (CC). This reference experiment, carried out in the same way as the titration with protein sample, was subtracted from the sample data. Reference corresponds to the signal between consecutive injections when no change in the heat flow is detected. Before subtracting the reference, the molar ratio was set similar for both set of data. Once subtraction was performed, the area data (ΔH) was fitted by ORIGIN software, using the “one set of sites” built-in curve fitting model. Fitting cycles were repeated until χ2 value was not reduced anymore and fitting parameters (N, K, ΔH, and ΔS) were obtained.
The usual binding curves plotted in kcal·mol−1 of injectant vs. the molar ratio (mol Ca·mol−1 of protein) will be presented in this study vs. the mass ratio (g Ca·g−1 of protein). Indeed, due to the complex mixture of milk proteins sample, this presentation will be more appropriate.
2.4. Electrophoretic mobility
Experiments similar to that of ITC were reproduced on a larger volume of milk protein solution to determine the electrophoretic mobility variations upon CC titration. This former parameter was determined by zeta-sizer (Malvern Instrument Ltd., Worcestershire, UK). Volumes of milk and CC titrated were scaled up to keep proportionality with ITC reaction cell. Electrophoretic mobility unit (μm cm·s−1·V−1) will be referred to as e.m.u. in the following sections.
2.5. Particle size determination
Experiments similar to that of ITC were reproduced on a larger volume of milk protein solution to determine the particle size variations upon CC titration. This former parameter was determined by nano-sizer (Malvern Instrument Ltd., Worcestershire, UK). Volumes of milk and CC titrated were scaled up to keep proportionality with ITC reaction cell.
3. RESULTS AND DISCUSSION
To the authors’ knowledge, few data concerning the thermodynamic characterization of Ca-milk protein interactions in a complex mixture of proteins are available. Calcium binding with purified milk proteins (i.e. αs-casein, β-casein, κ-casein, β-lactoglobulin, or α-lactalbumin) is often more studied in the literature [2, 5, 13, 14, 20, 21]. Hence, whole milk protein solution (prepared from industrial skim milk powder) was titrated with CC (50 mmol·L−1) at 25 °C to thermodynamically characterize Ca-protein interactions. Three different types of ITC experiments were carried out: (i) In the dilution experiments, water was injected into a protein solution. The signal obtained was considered negligible compared to those obtained with the two following experiments. (ii) In the binding titration, CC was injected into the protein solution (sample). (iii) Then, CC dilution into Milli-Q water (reference) was also measured to eliminate possible contribution to this signal, i.e. the so-called heat of dilution. In the analysis of our experiments, this latter signal was subtracted from the binding titration curve.
Once saturation is achieved, the binding enthalpy (ΔH, kcal·mol−1 of CC) is plotted as a function of the mass ratio (g Ca·g−1 of protein, Fig. 1). The binding parameters (N, K, ΔH, and ΔS) obtained from a “one set of sites” fitting model are summarized in Table I, and the quality of the fit was evaluated from the χ2 value. The “one set of sites” fitting model matched exactly with the experimental data. As a consequence, a very low χ2 value, indicative of the high quality of the fit, was obtained (mean value ~ 7.3).
 |
Figure 1. Titration of milk protein solution by CC (50 mmol·L−1) at 25 °C after subtraction of the reference binding curve (CC titration into Milli-Q water) – mean of duplicated experiments. |
Binding parameters obtained from a “one set of sites” fitting model – mean of duplicated experiments.
The titration of milk protein solution with CC shows a highly reproducible signal, which is of endothermic nature (ΔH > 0). The dilution enthalpy of milk proteins, observed once saturation is reached, measures the energy changes in the interaction of solvated solute species upon dilution. In our experiment, the enthalpy of dilution is negligible.
From the data in Figure 1, N is obtained at half saturation value and K corresponds to the slope of the binding curve. The whole milk protein fraction could bind up to 8 mg Ca·g−1 of milk protein at saturation. The binding constant (K) was set to 1.07 × 103 mol·L−1. The binding enthalpy (ΔH) per mole of cation (Ca2+) was endothermic (1910 cal·mol−1 CC) and the binding entropy (ΔS) was of 20.3 cal·mol−1·K−1 (Tab. I). Hence, at 25 °C (equivalent to 298 K), the Gibbs free energy (ΔG) was calculated from the following equation:
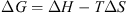 (1)
(1)
The results obtained in this study concerning the stoichiometry of Ca-milk protein interactions were compared with other investigations (Tab. II). In most of the studies, the various techniques used (centrifugation, ultrafiltration (UF), dialysis, etc.) separated the colloidal protein phase from the soluble phase. The Ca bound to proteins was calculated as the difference between the total Ca measured in the whole sample and the soluble Ca contained in the soluble phase.
Calcium-milk protein interaction determined by ITC – comparison with other investigations.
The results of this study carried out on whole milk proteins are in the range of the values (6.4–13.2 mg Ca·g−1 protein) obtained by previous authors on whole caseins [3, 13, 21, 29, 33] and more particularly on β-caseins [5]. The values of moles Ca bound per 105 g proteins (N) vary for each purified protein fraction (Tab. II). We could expect that differences observed in the given stoichiometric values come from variations in the method of protein sample preparation, in the protein fraction studied, and in the various physico-chemical conditions used (i.e. pH, T, the use of distinct buffers with different ionic strength). Indeed, the screen effect of charges by high ionic strength reduces Ca-protein interactions as it can be observed through a partial solubilization of Ca bound to proteins [18]. Furthermore, the effect of pH on the binding of Ca2+ to milk proteins is also reported in the literature [13, 14, 29, 33].
These variations can also be related to the experimental techniques used to determine the interaction (ITC, ion selective electrode, etc.).
Pappas and Rothwell [21] determined the total binding Ca capacity of different milk proteins using continuous UF to reach the binding equilibrium. At such an equilibrium, milk caseins had a high Ca-binding capacity due to the high number of Ca-binding sites, and the order of binding capacity was determined as αs1-casein > β-casein > κ-casein. For whey proteins, the order was set as β-lactoglobulin and α-lactalbumin. Former authors used the Klotz equation [15] to plot another variant of the Scatchard graph, where coordinates change a little, and to determine the maximum number of moles of Ca bound to 105 g of proteins. This representation involves that the plotting of the corresponding curve gives a linear relationship. Yet, the results obtained by former authors deviated from linearity for the whole casein sample. According to Pappas and Rothwell, this may be due to the electrostatic influence of the bound Ca on its subsequent binding and/or the variation in the type and the number of Ca-binding sites. Yet, similarly to our results, former authors reported that the binding data of whole casein obtained within the range of 10–20 mmol·L−1 of CC were in better agreement with linearity.
To evaluate the electrostatic influence upon Ca-milk protein interaction, Ca-binding titration was performed under the same conditions as that during ITC experiment and was followed by zeta-sizer. The CC titration lasted to reach a similar plateau value of electrophoretic mobility, as that observed in ITC experiment. The electrophoretic mobility of milk protein solution, initially of −1.95 e.m.u. increased to reach −1.56 e.m.u. at saturation (Fig. 2), and this was indicative of Ca-protein interactions of electrostatic nature.
 |
Figure 2. The electrophoretic mobility variations upon titration of milk protein solution by CC (50 mmol·L−1). |
The electrophoretic mobility variation upon CC titration is low (0.39 e.m.u.) and the binding of Ca on milk protein shows a highly endothermic signal (ΔH > 0). Hence, the exothermic part of the signal involved in the electrostatic interactions between Ca2+ and milk proteins is low and completely hidden by the strong endothermic signal. Sinn and co-workers studied the binding of Ca to polyelectrolytes [28] and more recently, to negatively charged phosphatidylcholine-phosphatidylserine membranes [27] by ITC. In both cases, former authors reported a strong endothermic reaction, enthalpy driven, with a gain in entropy. Furthermore, Dimova et al. [6] studied the binding of polymers to calcite crystals in water or Ca ion and also observed an overall endothermic phenomenon for the latter case solely. These results were unexpected since the electrostatic interactions are known to be highly exothermic. However, the endothermic character of this process can be understood in terms of competition between the polymer/ion, polymer/water, and ion/water interactions. Similarly to the former authors, we could suggest that not Coulomb interactions, but rather liberation of water molecules, either from the hydration shells of the Ca ion and/or dehydration of hydrophobic core of proteins was the driven energy source for the binding of multivalent ions onto milk proteins. Indeed, the binding of one Ca2+ ion to the polymer distorts the hydration shells of both binding partners and leads to the liberation of many water molecules from these shells. Before the binding reaction, free Ca2+ requires at least six water molecules to be fully solvated [8]. After binding Ca2+, at least seven water molecules are released from both Ca2+ (six water molecules) and protein (one water molecule replaced with Ca2+). Thus, the entropy increase upon Ca2+ binding is considered to be related to the release of water molecules and particularly from Ca2+. Roughly speaking, the energy gain associated with the binding of the Ca2+ ion to the polymer is overcompensated by the energy loss arising from the broken hydrogen bonds. This interpretation is in accordance with the study of Garidel and Blume [9], who reported that Ca ion binding involved a strong dehydration of hydrophobic regions.
As a consequence, binding should be rather described as a counterion H+/Ca2+ exchange, which is more energetically neutral (less exothermic phenomenon) than electrostatic forces.
Simultaneously to electrophoretic mobility measurement, the particle size of micelles was measured using nano-sizer (Fig. 3). The particle size decreased upon Ca titration and went on to diminish even when the electrophoretic mobility plateau value of −1.56 e.m.u. was already reached. The milk particle size measured varied from 215 to about 190 nm. This retraction of casein micelles could be interpreted as the absorption of Ca added either in the periphery of the casein micelles, i.e. onto the negatively charged hairy layer of κ-caseins, or in the core of the casein micelles involving the reinforcement of internal interaction forces. Yet, taking into account the composition in major amino acids involved in the interaction with Ca (i.e. Asp, Glu, and SerP) present in various caseins [7], the absorption of Ca would rather occur in the core of casein micelles. Indeed, κ-caseins are poorer in these specific amino acids. Furthermore, as described by Philippe et al. [22, 23] a phenomenon of dehydration of casein micelles occurs upon CC addition. Although former authors did not detect any average diameter variations at a lower range of CC concentration studied (0–13 mmol·kg−1), casein micelles dehydration should also explain the slight decrease of particle size.
 |
Figure 3. The particle size (nanometer) variations upon titration of milk protein solution by CC (50 mmol·L−1). |
Acknowledgments
The authors are indebted to Unilever Research and Development Center of Vlaardingen (The Netherlands) for their financial support and to the laboratory of RNA Maturation and Molecular Enzymology (MAEM, Nancy University, Faculty of Sciences) for ITC experiments.
References
- Anema S.G., Li Y., Re-equilibration of the mineral in skim milk during reconstitution, Milchwissenschaft 58 (2003) 174–178.
- Bingham E.W., Farrell H.M., Carroll R.J.,
Properties of dephosphorylated
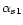 -casein.
Precipitation by calcium ions and micelle
formation, Biochemistry 11 (1972)
2450–2454 [CrossRef] [PubMed].
-casein.
Precipitation by calcium ions and micelle
formation, Biochemistry 11 (1972)
2450–2454 [CrossRef] [PubMed].
- Carr C.W., Studies on the binding of small ions in protein solutions with the use of membrane electrodes. III. The binding of chloride ions in solutions of various proteins, Arch. Biochem. Biophys. 46 (1953) 417–423 [CrossRef] [PubMed].
- Chanutin A., Ludewig S., Masket A.V., Studies on the calcium-protein relationship with the aid of ultracentrifuge. I. Observation on calcium-caseinate solutions, J. Biol. Chem. 143 (1942) 737–751.
- Dickson I.R., Perkins D.J., Studies on the interactions between purified bovine caseins and alkaline-earth metal ions, Biochemistry 124 (1971) 235–240.
- Dimova R., Lipowsky R., Mastai Y., Antonietti M., Binding of polymers to calcite crystals in water: characterization by isothermal titration calorimetry, Langmuir 19 (2003) 6097–6103 [CrossRef].
- Eigel W.N., Butler J.E., Ernstrom C.A., Farrell H.M., Harwalker V.R., Jenness R., Withney R.M., Nomenclature of proteins of cow's milk: fifth revision, J. Dairy Sci. 67 (1984) 1599–1631.
- Enderby J.E., Ions in aqueous solutions, Sci. Prog. 67 (1981) 553–573.
- Garidel P., Blume A., Interaction of alkaline earth cations with the negatively charged phospholipid 1,2-dimyristoyl-sn-glycero-3-phosphoglycerol: a differential scanning and isothermal titration calorimetric study, Langmuir 15 (1999) 5526–5534 [CrossRef].
- Gaucheron F., Interactions caséines-cations, in: Gaucheron F. (Ed.), Minéraux et produits laitiers, Lavoisier, Paris, France, 2004, pp. 81–112.
- Gopal B., Swaminathan C.P., Bhattacharya S., Bhattacharya A., Murthy M.R.N., Surolia A., Thermodynamics of metal ion binding and denaturation of a calcium binding protein from Entamoeba histolytica, Biochemistry 36 (1997) 10910–10916 [CrossRef] [PubMed].
- Hendrix T., Griko Y.V., Privalov P.L., A calorimetric study of the influence of calcium on the stability of bovine-lactalbumin, Biophys. Chem. 84 (2000) 27–34 [CrossRef] [PubMed].
- Imade T., Sato Y., Noguchi H., Interaction of calcium ion with bovine caseins, Agric. Biol. Chem. 41 (1977) 2131–2137.
- Jeyarajah S., Allen J.C., Calcium binding
and salt induced structural changes of native
and preheated
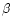 -lactoglobulin, J. Agric.
Food Chem. 42 (1994) 80–85 [CrossRef].
-lactoglobulin, J. Agric.
Food Chem. 42 (1994) 80–85 [CrossRef].
- Klotz I., The nature of some ion-protein complexes, Cold Spring Harbor Symposia Quant. Biol. 14 (1950) 97–112.
- Kuroki R., Nitta K., Yutani K., Thermodynamic changes in the binding of Ca2+ to a mutant human lysozyme (D86/92), J. Biol. Chem. 267 (1992) 24297–24301 [PubMed].
- Ladbury J.E., Chowdhry B.Z., Sensing the heat: the application of isothermal titration calorimetry to thermodynamic studies of biomolecular interactions, Chem. Biol. 3 (1996) 791–801 [CrossRef] [PubMed].
- Le Graët Y., Brulé G., Les équilibres minéraux du lait : influence du pH et de la force ionique, Lait 73 (1993) 51–60 [CrossRef].
- Nielsen A.D., Fuglsang C.C., Westh P., Isothermal titration calorimetric procedure to determine protein-metal ion binding parameters in the presence of excess metal ion or chelator, Anal. Biochem. 314 (2003) 227–234 [CrossRef] [PubMed].
- Noble R.W., Waugh D.F., Casein micelles. Formation and structure, J. Am. Chem. Soc. 87 (1965) 2236–2245 [CrossRef] [PubMed].
- Pappas C.P., Rothwell J., The effects of heating, alone or in the presence of calcium or lactose, on calcium binding to milk proteins, Food Chem. 42 (1991) 183–201 [CrossRef].
- Philippe M., Gaucheron F., Le Graët Y., Michel F., Garem A., Physicochemical characterisation of calcium-supplemented skim milk, Lait 83 (2003) 45–59 [CrossRef].
- Philippe M., Le Graët Y., Gaucheron F., The effects of different cations on the physicochemical characteristics of casein micelles, Food Chem. 90 (2005) 673–683 [CrossRef].
- Saboury A.A., Atri M.S., Sanati M.H., Moosavi-Movahedi A.A., Haghbeen K., Effects of calcium binding on the structure and stability of human growth hormone, Int. J. Biol. Macromol. 36 (2005) 305–309 [CrossRef] [PubMed].
- Saboury A.A., Atri M.S., Sanati M.H., Sadeghi M., Application of a simple calorimetric data analysis on the binding study of calcium ions by human growth hormone, J. Therm. Anal. Calorim. 83 (2006) 175–179 [CrossRef].
- Saboury A.A., Karbassi F., Thermodynamic studies on the interaction of calcium ions with alpha-amylase, Thermochim. Acta 362 (2000) 121–129 [CrossRef].
- Sinn C.G., Antonietti M., Dimova R., Binding of calcium to phosphatidylcholine-phosphatidylserine membranes, Colloids and Surfaces A: Physichochem. Eng. Aspects 282–283 (2006) 410–419 [CrossRef].
- Sinn C.G., Dimova R., Antonietti M., Isothermal titration calorimetry of the polyelectrolyte/ water interaction and binding of Ca2+: effects determining the quality of polymeric scale inhibitors, Macromolecules 37 (2004) 3444–3450 [CrossRef].
- Tessier H., Rose D., Heat stability of casein in the presence of calcium and other salts, J. Dairy Sci. 44 (1961) 1238–1246.
- Vanhooren A., Vanhee K., Noyelle K., Majer Z., Joniau M., Hanssens I., Structural basis for difference in heat capacity increments for Ca2+ binding to two á-lactalbumin, Biophys. J. 82 (2002) 407–417 [CrossRef] [PubMed].
- Volatier J.L., Maffre J., Couvreur A., Enquête individuelle et nationale sur les consommations alimentaires (INCA), Lavoisier, Paris, France, 2000.
- Yamniuk A.P., Vogel H.J., Calcium- and magnesium-dependent interactions between calcium- and integrin-binding protein and the integrin áIIb cytoplasmic domain, Protein Sci. 14 (2005) 1429–1437 [CrossRef] [PubMed].
- Zittle C.A., Della Monica E.S., Rudd R.K., Custer J.H., Binding of calcium to casein: influence of pH, calcium and phosphate concentrations, Arch. Biochem. Biophys. 76 (1958) 342–353 [CrossRef] [PubMed].
All Tables
Binding parameters obtained from a “one set of sites” fitting model – mean of duplicated experiments.
Calcium-milk protein interaction determined by ITC – comparison with other investigations.
All Figures
 |
Figure 1. Titration of milk protein solution by CC (50 mmol·L−1) at 25 °C after subtraction of the reference binding curve (CC titration into Milli-Q water) – mean of duplicated experiments. |
| In the text | |
 |
Figure 2. The electrophoretic mobility variations upon titration of milk protein solution by CC (50 mmol·L−1). |
| In the text | |
 |
Figure 3. The particle size (nanometer) variations upon titration of milk protein solution by CC (50 mmol·L−1). |
| In the text | |


