| Issue |
Dairy Sci. Technol.
Volume 89, Number 5, September-October 2009
|
|
|---|---|---|
| Page(s) | 511 - 518 | |
| DOI | https://doi.org/10.1051/dst/2009028 | |
| Published online | 19 August 2009 | |
Note
Bovine colostrum as substrate for the preparation of growth factor-enriched protein extracts: Identifying the optimal collection period during lactation制备富含生长因子的牛初乳蛋白提取物:泌乳期内最佳收乳时间的确定
Le colostrum bovin comme substrat pour la préparation d’extraits protéiques enrichis en facteurs de croissance : identification de la période optimale de collecte au cours de la lactation
Alicia Montoni1, Sylvie F. Gauthier1*, Caroline Richard1, Patrice E. Poubelle2, Yvan Chouinard1 and Yves Pouliot1
1 STELA Dairy Research Center and Institute of Nutraceuticals and Functional Foods (INAF), Université Laval, Quebec City, QC, G1V 0A6, Canada
2 Centre de recherche en Rhumatologie et Immunologie (CRCHUL), Quebec City, QC G1V 4G2, Canada
* Corresponding author (通讯作者): Sylvie.Gauthier@fsaa.ulaval.ca
Received: 28 November 2008
Accepted: 9 June 2009
A growing number of growth factor-enriched bioactive protein extracts derived from colostrum are proposed for the treatment of inflammatory and cutaneous diseases. However, growth factor concentrations peak in the first secretions after parturition and vary widely among cows. The aim of this study was to identify the optimal collection period for colostrum used to prepare growth factor-enriched protein extracts and to characterize the relationship between growth factor and protein contents in colostrum and in milk collected during early lactation. Lacteal secretions from five Holstein cows from the first milking (< 12 h) to the 21st day after parturition were analysed for total protein and growth factor (TGF-β2, TGF-β1 and IGF-I) contents. Although concentrations varied widely between cows, statistically significant decreases were obtained for all components during the early stages of lactation. The concentrations of each growth factor during lactation were highly correlated (R2 > 0.93) and were also correlated with the total protein content (R2 > 0.90). The greatest decreases in the concentrations of the three growth factors (78–84%) were observed within 24–48 h after parturition, while values stabilized in mature milk. However, relative to protein, these decreases appeared much smaller (37–45%). Overall, these results suggest that the first 48 h after parturition would be optimal for harvesting colostrum for the production of growth factor-enriched protein extracts.
摘要
源于牛初乳的富含生长因子的活性蛋白提取物在治疗炎症和皮肤疾病中的显著疗效引起了人们广泛关注。然而,在泌乳初期分泌生长因子浓度的高峰值在奶牛个体之间变化幅度非常大。本研究目的是确定用于制备富含生长因子的牛初乳的最佳采乳时间,并且确定生长因子与初乳和泌乳初期原料乳中蛋白质含量之间的关系。分析了5 头荷斯坦奶牛(分娩后12 h到 21 天)乳样中生长因子(TGF-2, TGF-1, IGF-I)和蛋白质含量。在5头奶牛之间,所有分析指标的变化幅度较大,但是,所有指标在整个泌乳初期内随着时间的增加而显著降低。而且在整个泌乳期内,每种生长因子的浓度之间(R2 > 0.90)及与蛋白质浓度之间(R2 > 0.90)具有非常高的相关性。在泌乳后的24 h ~ 48 h 内三种生长因子浓度降低的幅度非常大(78% ~ 84%),但是在常乳中生长因子浓度变化不大。蛋白质浓度的变化幅度较小(37% ~ 45%)。综上所述,如果生产富含生长因子的蛋白提取物,泌乳后48 h 内是采集牛初乳的最佳时间。
Résumé
Un nombre croissant d’ingrédients protéiques bioactifs à base de facteurs de croissance issus du colostrum sont proposés pour le traitement des maladies inflammatoires et cutanées. Cependant, l’utilisation du colostrum comme matière première est limitée par l’apparition d’une forte concentration de ces molécules dans les premières sécrétions suivant le vêlage et par la grande variabilité de leur concentration entre les animaux. Le but de la présente étude était d’identifier la période optimale de collecte du colostrum pour la préparation d’extraits protéiques enrichis en facteurs de croissance et de caractériser la relation entre le contenu en facteurs de croissance et en protéines d’échantillons de colostrum et de lait collectés en début de lactation. Les sécrétions lactées de cinq vaches Holstein ont été collectées de la première traite (< 12 h) jusqu’au 21e jour suivant le vêlage, puis analysées pour leur teneur en protéines totales et en facteurs de croissance (TGF-β2, TGF-β1, IGF-I). Malgré la grande variabilité de la concentration de ces composantes observée entre les vaches, des différences statistiquement significatives ont été observées dès le début de la lactation. Les diminutions dans la concentration en facteurs de croissance des sécrétions lactées en cours de lactation étaient fortement inter-reliées (R2 > 0,93), mais aussi corrélées (R2 > 0,90) avec la teneur en protéines totales des échantillons. Les diminutions les plus élevées dans la concentration des trois facteurs de croissance (78–84 %) ont été observées après 24–48 h suivant le vêlage, pour atteindre des valeurs stables dans le lait mature. Cependant, lorsque les concentrations en facteurs de croissance étaient exprimées sur base protéique, ces diminutions étaient considérablement réduites (37–45 %) au cours de la même période. Les résultats suggèrent que les premières 48 h suivant le vêlage constituent la période optimale pour la collecte du colostrum en vue de la production d’ingrédients protéiques enrichis en facteurs de croissance.
Key words: bovine colostrum / bovine milk / growth factor / IFG-I / TGF-β1 / TGF-β2 / growth factor-enriched ingredient
关键字 : 牛初乳 / 牛乳 / 生长因子 / IFG-I / TGF-β1 / TGF-β2 / 富含生长因子的配料
Mots clés : colostrum bovin / lait bovin / facteurs de croissance / IFG-I / TGF-β1 / TGF-β2 / ingrédients enrichis en facteurs de croissance
© INRA, EDP Sciences, 2009
1. INTRODUCTION
Colostrum, the first lacteal secretion of the mammary glands following calving, is known to provide passive immunity and all the nutrients essential to the development of the newborn calf [3]. It contains immunoglobulins and other antimicrobial factors, growth factors, cytokines, hormones and other biologically active molecules [10, 20]. The growth factors found in significant amounts in colostrum are transforming growth factors-beta 2 (TGF-β2) and insulin-like growth factor-I (IGF-I), for which wide concentration variations (150−1500 and 49–2000 ng·mL−1, respectively) have been reported [8]. The physiological effects of TGF-β-related growth factors and IGFs in colostrum and milk have been reviewed by Pakkanen and Aalto [15] and Gauthier et al. [8]. Several health-related applications of milk growth factors have been proposed such as for skin disorders, gut health, bone health and others [17]. Characterization of the numerous protein components in bovine colostrum, milk and whey has been done recently using the proteomic approach [7, 19], confirming the diversity of molecules present in these products. These new data are likely to stimulate the development of new colostrum-based bioactive protein ingredients. However, the known variability of colostrum composition during the first hours of lactation still represents a major challenge in the commercial-scale production of colostrum-based ingredients.
Technological approaches to prepare bioactive extracts containing growth factors found in bovine colostrum, milk or whey are growing in number [17]. Maubois et al. [13] proposed a combination of acidification/heat treatment of native (unheated) whey to precipitate a TGF-β2-rich fraction that was further recovered by tangential microfiltration. This method also enabled to fractionate whey proteins but was not applicable to bovine colostrum due to the presence of caseins that precipitate at their isoelectric point. Piot et al. [16] showed the potential of microfiltration for the removal of caseins from colostrum, while Lachkar et al. [11] proposed complexing with polysaccharides as an alternative approach of preparing a TGF-β2-enriched fraction from bovine colostrum. Recent work by Akbache et al. [1] has suggested that ultrafiltration/microfiltration membranes can be used to concentrate TGF-β2 and IGF-I from whey.
Despite the recent technological advances, only a few studies have focussed on the variability of colostrum composition as a function of time during early lactation. Elfstrand et al. [6] investigated compositional changes in growth factors in pooled colostrum collected 0–80 h postpartum from Swedish Friesian cows, demonstrating a rapid decrease in TGF-β2 and IGF-I within the first 24 h. In a recent study, Purup et al. [18] showed that the concentration of these growth factors decreased during lactation in Holstein Friesian cows. These authors also showed that whey separated from colostrum and milk stimulated growth of a human intestinal cell line to different extents depending on the stage of lactation. Changes in growth-promoting activity with stage of lactation were also observed and were attributed to changes in concentrations of minor bioactive compounds such as growth factors.
The aim of this study was to identify the optimal collection period for obtaining the starting material suitable for the preparation of growth factor-enriched protein extracts from colostrum. Although it is known that growth factor concentrations decrease during lactation, values published for bovine colostrum and milk vary to a large extent, probably because they are never expressed relative to protein content [8]. The aim of this study was therefore also to characterize the relationship between the growth factors and the total protein content of colostrum and milk collected during early lactation.
2. MATERIALS AND METHODS
2.1. Animals and sampling
Colostrum samples (total lacteal secretion) collected within 24 h from 14 Holstein cows aged 23–110 months (primapara or 2–7-calf multipara heifers) at the Laval University Animal Sciences Research Center (Deschambault, QC, Canada) were analysed for total protein, TGF-β2, TGF-β1 and IGF-I contents. In a second phase, five cows were selected for a 21-day sampling experiment. These were 24–81 months old and multipara (2–4 calves) or primapara in one case. They were housed in a tie-stall barn and given individually a single feeding per day ad libitum in the morning. The diet consisted of a total mixed ratio formulated to meet the National Research Council recommendations for transition cows [14], containing alfalfa silage, corn silage, grass hay, cracked corn, rolled barley, beet pulp and commercial energy (Energina, Agribrands Purina Canada Inc., Saint-Hubert, QC, Canada) and protein supplements (B-Top 45, Aliments Breton Inc., Saint-Bernard, QC, Canada).
The first colostrum sample was collected as soon as possible after parturition (< 12 h), followed by twice-daily collections (approximately at 8 and 17 h) for the next six days. Additional samples were collected from each cow on the 7th, 14th and 21st days postpartum once in the morning. All samples were immediately frozen and kept at −18 °C.
2.2. Defatting of colostrum and milk
Colostrum samples (1 L) were thawed overnight at 4 °C and then for 2–3 h at room temperature until entirely liquid. They were skimmed by centrifugation at 10 000× g for 30 min at 4 °C (Sorvall model RC-5B, GSA rotor, DuPont Instruments, Boston, MA, USA) and the solid fat layer was then carefully removed manually. The defatted colostrum samples were refrozen without delay at −20 °C until analysis.
2.3. Protein analysis
The total protein concentration was determined by the Dumas combustion method [9] using a FP-528 LECO apparatus (LECO Corp., Saint Joseph, MI, USA). The calibration standard curve was prepared using ammonium sulphate (99.99% w/w, Sigma-Aldrich, Oakville, ON, Canada) and the nitrogen content was converted to protein using the milk conversion factor (6.38).
2.4. Growth factors quantification
IGF-I, TGF-β1 and TGF-β2 contents were determined by enzyme-linked immunosorbent assay (ELISA) using the methodology described by Ben Ounis et al. [2], except for adjusting the sample dilutions to the range of growth factor concentrations in colostrum and milk.
Absorbance readings were performed at 450 nm for the three growth factors using a spectrophotometer plate reader (MultiSkan Spectrum model, Thermolabsystems, Brussels, Belgium). The assays were performed in triplicate (three wells per sample). The detection limit (DL) of the ELISA tests was established at 0.031 ng·mL−1 for TGF-β1 and TGF-β2, and at 0.023 ng·mL−1 for IGF-I.
2.5. Statistical analysis
The concentrations of total proteins and the three growth factors in colostrum and milk collected during the lactation period were submitted to statistical analysis using one-way analysis of variance and paired t tests between two consecutive samplings using SAS version 9.1. Also, linear regression analysis was performed on the total protein, TGF-β2, TGF-β1 and IGF-I concentrations for each colostrum and milk sample over the lactation period in order to establish their mutual coefficients of determination (R2).
3. RESULTS
The concentrations of total proteins, TGF-β2, TGF-β1 and IGF-I in colostrum collected during the first day postpartum (< 24 h) were first determined for 14 individual cows. A wide range of values were obtained for all components. Total proteins ranged from 80.3 to 220.1 mg·mL−1 (mean ± SD of 155.2 ± 40.9), whereas TGF-β2, TGF-β1 and IGF-I ranged from 73 to 436 ng·mL−1 (mean ± SD of 213.9 ± 21.0), from 9 to 56 ng·mL−1 (mean ± SD of 20.6 ± 2.8) and from 4 to 247 ng·mL−1 (mean ± SD of 90.9 ± 11.8), respectively. No significant statistical correlation could be established (data not shown) between the concentration of these components in the colostrum and the cow age or number of calvings.
Given the high variability observed in the colostrums collected from the 14 cows during the first day postpartum (< 24 h), a second experimental phase including a smaller number of cows (n = 5) was designed in order to characterize the changes in total proteins, TGF-β2, TGF-β1 and IGF-I from the first milking after parturition (< 12 h) to the 21st day postpartum. As shown in Figure 1, wide variability was observed among the cows for all components analysed, especially during the first 48 h period, as indicated by the error bars. However, a paired t test statistical analysis of the data showed significant decreases already at 12 h (P < 0.05) and 24 h (3rd milking, P < 0.01; 4th milking, P < 0.001) for TGF-β1, while significant decreases in total proteins (3rd milking, P < 0.05; 4th milking, P < 0.01), IGF-I (3rd and 4th milkings, P < 0.05) and TGF-β2 (3rd milking, P < 0.01; 4th milking, P < 0.05) occurred only at 24 h. A smaller but significant (P < 0.05) decrease also occurred for TGF-β2 on the 4th day. This figure shows that the first 48 h after parturition represent the most critical period with respect to the growth factor content of colostrum.
 |
Figure 1. Evolution of total protein, IGF-I, TGF-β1 and TGF-β2 concentrations in the first milking (< 12 h) colostrum and milk collected from five Holstein cows over a 21-day post-parturition period. Values are reported as mean ± SD of the mean and significant differences between two consecutive values are indicated (*P < 0.05; **P < 0.01; and ***P < 0.001). |
In view of the similarities of the time-dependent decreases for the components investigated (Fig. 1), coefficients of determination (R2) were calculated by linear regression between the growth factors and also between each growth factor and the total protein content for each cow during the lactation period (data not shown). The calculated R2 values were higher than 0.90 for all three growth factors (0.93–0.95) and for their comparisons with total proteins (0.90–0.92), indicating that the growth factor contents of the lacteal secretions for 21 days postpartum were directly related to total protein content, even though the amount of growth factor was proportionally very low compared to protein (Fig. 1).
From the trends observed in Figure 1, the compositional data were grouped in five stages of lactation, namely first (< 12 h) and second milkings (12 h), 3rd to 6th milkings (24–48 h), transition milk (3–6 days) and mature milk (7–21 days). The relative changes (%) in total proteins and growth factors for each stage of lactation (data not shown) indicated that the TGF-β2, TGF-β1 and IGF-I concentrations in colostrum decreased by 78–84% from the initial value within the first 48 h. Although a major decrease (67%) in total protein concentration was also observed during this period, the subsequent decrease during the lactation period (3–21 days) was much less than for the three growth factors.
Table I presents growth factor contents relative to total protein for colostrum or milk collected during the five stages of lactation, along with the TGF-β2/TGF-β1 and TGF-β1 + TGF-β2/IGF-I concentration ratios. These values are important from the perspective of industrial production of protein extracts rich in growth factors. The data show that TGF-β2, TGF-β1 and IGF-I contents decreased, respectively, from 1799 to 1131 ng·g protein−1, from 115 to 63 ng·g protein−1 and from 568 to 321 ng·g protein−1 during the < 12–48 h period, corresponding to a reduction of 37–45% in growth factor contents of the protein extract. This reduction is much lower than those based on liquid volume (78–84%). The TGF-β2/TGF-β1 ratio varied between 15.6 and 18.0 during the same period (< 12–48 h), while TGF-β1 + TGF-β2/IGF-I varied between 3.1 and 3.7, indicating that the protein-based composition did not vary to a great extent during this period. However, the TGF-β1 + TGF-β2/IGF-I ratio increased to 9.0 during the 3–6 days period, mainly as a result of a major decrease in IGF-I.
Growth factor concentrations relative to protein and ratios of protein extracts at different stages of lactation.
4. DISCUSSION
The compositional data obtained in the present study are in good agreement with those published by Purup et al. [18] and Elfstrand et al. [6] on the early changes postpartum in total protein and TGF-β2 contents, despite the difference in animal breed. The main discrepancy was for IGF-I, which was 5- to 10-fold lower in this study, compared to Elfstrand et al. [6] who used radioimmunoassay. TGF-β1 was not determined by Elfstrand et al. [6], but our data are very close to those of Purup et al. [18].
The changes in total protein concentrations reported in this study are also in good agreement with those reported by Božanić [5], Blum and Hammon [4] and Levieux and Ollier [12]. As noted by Gauthier et al. [8], the marked decrease in total protein concentration during the first four days of lactation is generally not taken into account in published growth factor concentration data. The strong correlations (R2 > 0.90) found between the three growth factors and the total protein contents in our study suggest that growth factor concentrations in colostrum and milk should be expressed per gram of protein and that this would probably reduce some of the discrepancies between the published data and thus facilitate their comparison.
The growth factor contents found in colostrum and milk collected during the first month postpartum suggest that the first 12 h should be targeted to maximize concentration in growth factor-enriched ingredients, although the compositional data are still variable. In fact, the first milking postpartum would represent the ideal condition. However, as seen in Table I, when expressed relative to protein, the decreases in TGF-β2, TGF-β1 and IGF-I during the first 48 h are 37%, 45% and 43%, respectively, compared to the 78–84% range previously found when expressed per volume of liquid (Fig. 1). Moreover, the relative proportions of TGF-β2, TGF-β1 and IGF-I in colostrum protein-based extracts would not vary to a large extent within the first 48 h of lactation. It is of interest that should the purpose of the collection be to produce a TGF-β-enriched ingredient with the lowest amount of IGF-I, the optimal collection period might be days 3–6, when the TGF-β1 + TGF-β2/IGF-I ratio reaches 9.0 (Tab. I). This results from the fact that the IGF-I concentration is lowest, while TGF-β2 and TGF-β1 contents are low but still found at a useful concentration.
5. CONCLUSION
The results from this study underline the technological and economical challenges of using colostrum as a starting material for the production of growth factor-enriched protein extracts. Collecting and processing separately the first colostrum postpartum (< 12 h) would be difficult to achieve in practice because of the low volumes of colostrum produced. As a consequence, most commercial-scale colostrum producers are currently pooling milkings up to 24 h and those up to 48 h separately. However, our results suggest that, expressed relative to protein, the growth factor content of colostrum would be optimal during the first 48 h after parturition.
Acknowledgments
This work was supported by a Cooperative Research and Development (CRD) grant from the Natural Sciences and Engineering Research Council of Canada (NSERC) and Advitech Inc. The authors thank the Deschambault Research Center for the collaboration of their staff with this work and L. Troquier for her technical assistance with the analysis of samples.
References
- Akbache A., Lamiot E., Moroni O., Turgeon
S.L., Gauthier S.F., Pouliot Y., Use of
membrane processing to concentrate TGF-
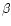 2 and
IGF-I from bovine milk and whey,
J. Membr. Sci. 326 (2009) 435–440 [CrossRef].
2 and
IGF-I from bovine milk and whey,
J. Membr. Sci. 326 (2009) 435–440 [CrossRef].
- Ben Ounis W., Gauthier S.F., Turgeon S.L., Roufik S., Pouliot Y., Separation of minor protein components from whey protein isolates by heparin affinity chromatography, Int. Dairy J. 18 (2008) 1043–1050 [CrossRef].
- Blum J.W., Nutritional physiology of neonatal calves, J. Anim. Physiol. Anim. Nutr. 90 (2006) 1–11 [CrossRef].
- Blum J.W., Hammon H., Colostrum effects on the gastrointestinal tract, and on nutritional, endocrine and metabolic parameters in neonatal calves, Livest. Prod. Sci. 66 (2000) 151–159 [CrossRef].
- Božanić R., [Importance and utilization of colostrum], Mljekarstvo 54 (2004) 209–224 (in Croatian).
- Elfstrand L., Lindmark-Månsson H., Paulsson M., Nyberg L., Åkesson B., Immunoglobulins, growth factors and growth hormone in bovine colostrum and the effects of processing, Int. Dairy J. 12 (2002) 879–887 [CrossRef].
- Fong Y.B., Norris C.S., Palmano K.P., Fractionation of bovine whey proteins and characterisation by proteomic techniques, Int. Dairy J. 18 (2008) 23–46 [CrossRef].
- Gauthier S.F., Pouliot Y., Maubois J.L., Growth factors from bovine milk and colostrum: composition, extraction and biological activities, Lait 86 (2006) 99–126 [CrossRef].
- IDF, Determination of nitrogen content – routine method using combustion according to the Dumas principle, Int. Dairy Fed., Brussels, Belgium 186 (2002).
- Korhonen H., Pihlanto A., Technological options for the production of health-promoting proteins and peptides derived from milk and colostrum, Curr. Pharm. Des. 13 (2007) 829–843 [CrossRef] [PubMed].
- Lachkar D., Lamiot E., Turgeon S.L., Gauthier S.F., Paquin P., Pouliot Y., An experimental approach for removing caseins from bovine colostrum using anionic polysaccharides, Int. J. Dairy Sci. 61 (2008) 43–50 [CrossRef].
- Levieux D., Ollier A., Bovine immunoglobulin
G,
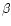 -lactogobulin,
-lactogobulin, 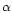 -lactalbumin and
serum albumin in colostrum and milk during
the early post partum period, J. Dairy Res.
66 (1999) 421–430 [CrossRef] [PubMed].
-lactalbumin and
serum albumin in colostrum and milk during
the early post partum period, J. Dairy Res.
66 (1999) 421–430 [CrossRef] [PubMed].
- Maubois J.L., Fauquant J., Jouan P., Bourtourault M., Method for obtaining a TGF-beta enriched protein fraction activated form, protein fraction and therapeutic applications, PCT Pat. No. WO 03/006500 (2003).
- National Research Council, Nutrient Requirements of Dairy Cattle, 7th revised edn., National Academy Press, Washington, DC, USA (2001) 184–213.
- Pakkanen R., Aalto J., Growth factors and antimicrobial factors of bovine colostrums, Int. Dairy J. 7 (1997) 285–297 [CrossRef].
- Piot M., Fauquant J., Madec M.N., Maubois J.L., Preparation of serocolostrum by membrane microfiltration, Lait 84 (2004) 333–341 [CrossRef].
- Pouliot Y., Gauthier S.F., Milk growth factors as health products: some technological aspects, Int. Dairy J. 16 (2006) 1415–1420 [CrossRef].
- Purup S., Vestergaard M., Pedersen L.O., Sejrsen K., Biological activity of bovine milk on proliferation of human intestinal cells, J. Dairy Res. 74 (2007) 58–65 [CrossRef] [PubMed].
- Smolenski G., Haines S., Kwan F.Y., Bond J., Farr V., Davis S.R., Stelwagen K., Wheeler T.T., Characterisation of host defence proteins in milk using a proteomic approach, J. Proteome Res. 6 (2007) 207–215 [CrossRef] [PubMed].
- Stelwagen K., Carpenter E., Haigh B., Hodgkinson A., Wheeler T.T., Immune components of bovine colostrum and milk, J. Anim. Sci. 87 (Suppl. 1) (2009) 3–9 [CrossRef] [PubMed].
All Tables
Growth factor concentrations relative to protein and ratios of protein extracts at different stages of lactation.
All Figures
 |
Figure 1. Evolution of total protein, IGF-I, TGF-β1 and TGF-β2 concentrations in the first milking (< 12 h) colostrum and milk collected from five Holstein cows over a 21-day post-parturition period. Values are reported as mean ± SD of the mean and significant differences between two consecutive values are indicated (*P < 0.05; **P < 0.01; and ***P < 0.001). |
| In the text | |


